|
||||||||||
|
||||||||
|
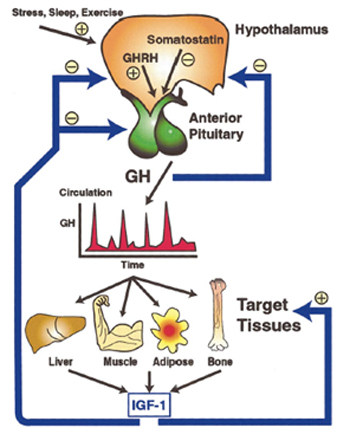
1. What is growth hormone? Growth hormone (GH) is a single polypeptide hormone secreted from the cells of the pituitary gland. GHs in human are present as isomers of 27, 22, 20, 17, and 5 kDa. GH of 22 kDa, called somatropin, is the major component of GHs produced by human pituitary and used for the therapy. This kind of GH is composed of 191 amino acids and contains four α-helices arranged in a left-handed bundle orientation. Two disulfide bridges are located at Cys53-Cys165 and Cys182-Cys189 in GH, and the Cys residues make up the loop structure of the GH molecule. The main effect of GH is to promote postnatal longitudinal growth. Hyposecretion of GH can lead to dwarfism. The growth-promoting effects of GH result from GH’s diverse and pleiotropic effects on cellular metabolism and differentiation. GH is known to regulate the lipid, carbohydrate, nitrogen, and mineral metabolism within a cell. Many of GH actions are mediated by the activation of insulin-like growth factor I (IGF-1). GH produced using recombinant DNA technology was approved by FDA for the treatment of children in short status in 1980s. Only the recombinant human GH (rhGH) has been used for the therapy since 1985, following the reports of Creutsfeldt-Jakob disease in four patients administered with pituitary-derived hGH. GH is also used for the treatment of adults with GH deficiency (GHD).
1) Manufacturing Process “Novel human growth hormone releasing factor(hGRF)-human epidermalgrowth factor fusion gene, and its expression vector and manufacturing process of hGRF” Registered: Korea (KR 1999-0009995) 2) Liquid Formulation “A stable liquid formulation of human growth hormone” - Registered: Korea (KR 2005-0046381) - Pending: PCT, China, Europe, Japan, USA, Brazil, India, Russia 3) Pen-type Syringe “Medicines injectors” A patent is being processed for application in Korea, and Taiwan 4. Key Benefits and Features 1) Convenience of Administration - Liquid formulation with pen-type syringe - Various dosage: 4 IU, 16 IU, 22 IU 2) Proven Efficacy - The efficacy of Caretropin™ is superior to Eutropin™ (LGLS) - Effective for the reduction of abdominal obesity in adults 3) High Quality & Competitive Price - Compliance with European Pharmacopoeia standards - High production yield using patent vector system 
|
 > Products > CARETROPIN
> Products > CARETROPIN